GENOMICS AND BIOINFORMATICS

Globally, the demand for genomics workflows is growing exponentially. The Hugh Green Cytometry Centre is responding to this demand by expanding its genomics capabilities.
GENOMICS
We currently house a BD Rhapsody Express Instrument for preparing single-cell cDNA libraries for single-cell RNA sequencing workflows that allows us to sequence up to 20,000 cells simultaneously. The Hugh Green Cytometry Centre also has an Oxford Nanopore MinION sequencer that allows us to obtain sequencing results where long reads are essential or beneficial.
Single-cell RNA sequencing libraries are prepared as a service. Sample preparation is carried out by the individual researcher and we offer our support in experimental design for optimal outcomes. Our high-end cell sorting capabilities allow us to assist you in selecting only the cell subset of interest, thereby substantially reducing sequencing costs.
We have a dedicated space for in-house molecular workflows and library generation. Illumina sequencing is currently performed externally.
BIOINFORMATICS
Bioinformatics is the process of converting data-rich research outputs into something that can be better understood and interpreted. Bioinformaticians help people to interpret information, most typically very large sources of information on the order of hundreds of gigabytes to terabytes of data.
Bioinformatics can be considered as the intersection of biology, computer science, and maths or statistics. Most bioinformaticians have expertise in only one or two of these areas, so rely on the understanding of other researchers to help provide the context to interpret and process data.
Bioinformatics services in the form of analysis support, software use, pipeline establishment, and teaching are available for data collected using the our genomics platforms (BD Rhapsody and MinION). Please approach us with your specific needs and we will determine how we can help.
Our aim is to create a seamless pipeline for cell analysis, where genomic data can be generated in-house to be processed, mapped and contextualised with disease models as well as gene and protein expression data from flow cytometry and/or microscopy.
Here are a set of figures that were generated by our bioinformatician Dr David Eccles:

Visualization of single cell RNA-seq data from migratory dendritic cells in the skin of wild type (C57) or STAT6 knock out mice using Uniform Manifold Approximation and Projection (UMAP). Dendritic cells from skin draining lymph nodes were sorted using a BD INFLUX™ Cell sorter loaded on BD Rhapsody™ Single-Cell Analysis System and analyzed using the BD Rhapsody™ WTA Analysis Kit. Mayer J. et al. Nat Immunol 2021.
TECHNOLOGIES
Digital Droplet PCR
The Hugh Green Cytometry Centre offers digital droplet PCR (ddPCR) with the BIORAD QX200 Droplet Digital PCR System. The method is based on water-oil emulsion droplet technology. A sample is fractionated into 20,000 droplets, and PCR amplification of the template molecules occurs in each individual droplet. ddPCR technology uses reagents and workflows similar to those used for most standard TaqMan probe-based assays. The massive sample partitioning is a key aspect of the ddPCR technique.
DdPCR is also ideal when PCR inhibitors might be present (eg in faecal samples). The ddPCR has been successfully employed in wastewater testing for COVID-19 where template quality and quantity is low and PCR inhibitors are abundant. A big advantage compared to qPCR is that a standard curve is not needed, as ddPCR counts template molecules and gives an absolute measure of concentration.
The ddPCR System can be used to:
-
Detect rare DNA target copies
-
Determine copy number variation
-
Measure gene expression levels

Digital droplet PCR machine
Applications of the QX200 Droplet Digital PCR System include:
-
Cancer biomarker studies and copy number variation
Measure varying degrees of cancer mutations, detect rare DNA target copies, and resolve copy number variation states. PrimePCR ddPCR Assays are now available for mutation and copy number detection. These are predesigned, wet-lab validated assays.
-
Pathogen detection
Quantify small fold changes in target DNA or RNA molecules in pathogen detection and monitoring.
-
Next generation sequencing
Perform accurate quantification and qualification of NGS libraries without the use of a standard curve.
-
Gene expression analysis
Achieve reliable and reproducible measurements of small fold changes for low abundance of mRNA and miRNA.
-
Environmental monitoring
Test a wide variety of environmental samples like soil and water.
-
Food testing
Perform routine evaluation of genetically modified organisms (GMO) using validated ddPCR methods.
Single Cell RNA Sequencing with the BD Rhapsody System
The Hugh Green Cytometry Centre houses a BD Rhapsody Express for capture of single cells for single cell RNA sequencing via a gentle microwell capture procedure. Libraries are prepared and QCed in house before they are sent out to a sequencing provider of choice. Data analysis can be supported by our bioinformatics staff or performed independently by the client.
BD Rhapsody Express is coupled with the optional data QC by Seven Bridges. SeqGeq, a desktop bioinformatics platform that makes complex scRNA seq analysis accessible with an intuitive interface, is recommended by BD for data analysis.

Nanopore Sequencing with the MinION
Nanopore sequencing involves the translocation of a long polymer through a protein pore with the help of an electric current which detects changes in translocation speed as it goes through. The changes are represented as the difference in electric current over time. The speed of translocation usually depends on the size and shape of the substance going through the pore, so the current can be used as a proxy for electrophysical properties. By understanding how that shape changes with different DNA bases, it's possible to work backwards from the electrical current trace to the bases. After one read finishes, another can feed into the nanopore, resulting in a very fast, observational method of DNA sequencing.
We have a MinION sequencer, which can carry out nanopore sequencing using a consumable flow cell. On the bottom of the flow cell is an electrical current sensing circuits and circuits to enable the transfer of those electrical signals over a USB connection.
On the top of the flow cell is an array of 2048 sequencing wells. Each well is covered with a delicate, fluid-impermeable polymer membrane, and embedded into the membrane are purpose-built (or more correctly, purpose-mutated) protein pores that are a few tens of nanometres across. An electrical potential is set up across either side of the membrane, which encourages the flow of ions through the nanopore. If the sequencer is working well, then most of the ions flowing through are the polymer templates (usually DNA) that you're trying to sequence.
512 of these wells can be hooked up to the sensing circuits at any one time, and those circuits will take snapshots of the electrical state of the wells at 4 kHz: 4 thousand snapshots per second, measuring up to 450 DNA bases per second, with a maximum output of about 40 gigabases from a two-day sequencing run.
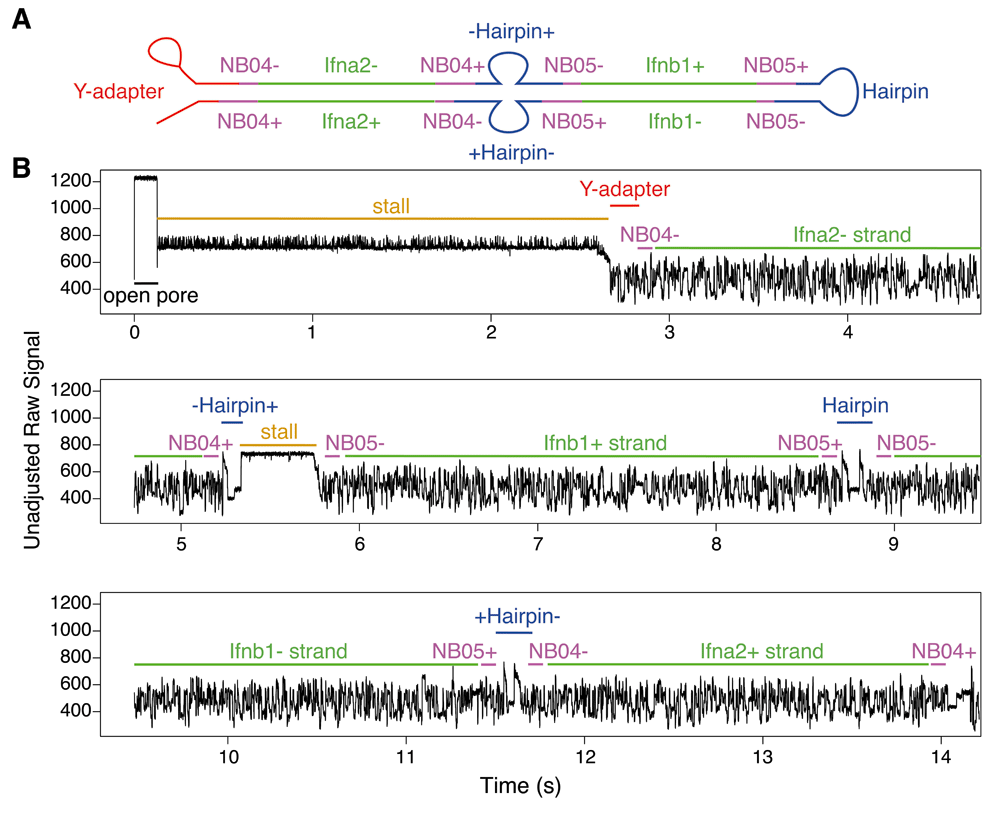
A symbolic representation of a chimeric read captured by an ONT MinION DNA sequencer (A), together with the annotated raw signal (B), containing three separate hairpin events within the same base-called sequence. Hairpin features can be seen by eye within the signal; attached barcodes demonstrate that this sequence was formed from an overnight ligation reaction. White R., et al. F1000 Research 2017.

